
Soligenix is a late-stage biopharmaceutical company focused on developing and commercializing products to treat rare diseases where there is an unmet medical need. Our Specialized BioTherapeutics business segment is developing and moving toward potential commercialization of HyBryte™ (SGX301 or synthetic hypericin sodium) as a novel photodynamic therapy utilizing safe visible light for the treatment of cutaneous T-cell lymphoma (CTCL). With successful completion of the second Phase 3 study, regulatory approvals will be sought to support potential commercialization worldwide.

INTERVIEW WITH CHRIS SCHABER FROM 3/26/2025
WSA caught up with Dr. Chris Schaber to discuss progress on their CiVax booster vaccine as well as year end 2024 Financial results and accomplishments:
INTERVIEW WITH CHRIS SCHABER , PhD, CEO AND PRESIDENT FROM 12/16/24
INTERVIEW TRANSCRIPTS:
WSA: Good day from Wall Street. This is Juan Costello, senior analyst with the Wall Street Analyzer, joining us today is Chris Schaber PhD. He is the CEO and President at Soligenix Incorporated—The company trades on Nasdaq, ticker symbol SNGX. Thanks for joining us today there, Chris.
Chris Schaber: Juan, thank you for having me. It’s a pleasure.
WSA: Yeah, great. It’s definitely been a while around the pandemic time. And for some of our listeners that may not have caught our previous calls, can you provide us an intro and overview there of the company?
Chris Schaber: Sure. Soligenix as you pointed out, is a publicly traded company. We are a late clinical stage biopharmaceutical company focused on developing and commercializing products to treat rare diseases and areas of unmet medical need. And I think what makes us unique is the fact that we’ve divided our business into two segments. A specialized biotherapeutics segment focused on small molecules in oncology and inflammation, and a separate public health solutions business segment that’s focused predominantly on heat stable vaccines for bio threats and emerging infectious diseases like Ebola and Marburg. This segment of our business has been funded entirely by the US government to date. To date, we’ve received in excess of $60 million of non-dilutive government funding. So it’s really allowed us to build out a more robust pipeline than you would typically see for a company our size and market cap, and provides for multiple shots on goal to mitigate risk.
WSA: Yeah, great. And so can you bring us up to speed on some of the most recent news there, including that you just formed a European Medical Advisory Board for your Cutaneous T-cell lymphoma study?
Chris Schaber: Yes, so there is a lot going on. Multiple studies ongoing or ready to initiate. We have, as you pointed out, have formed a European Medical Advisory Board that’s focused around HyBryte™ (synthetic hypericin) Cutaneous T-cell lymphoma program. In this particular program, will be initiating a confirmatory Phase 3 study. We completed one Phase 3 study already that was successful, published in JAMA Dermatology, a very well-known peer reviewed journal in the space. And we’ll now be embarking on that second Phase 3 study; next month is our target. In addition, we have two studies that are ongoing: One in psoriasis that uses the same active ingredient as HyBryte, that’s actively enrolling and another study in Behçet’s disease, using a separate molecule that’s ongoing and actively enrolling. We expect top-line results for both in the first half of next year. And the Phase 3 study with HyBryte in cutaneous T-cell lymphoma, a rare and chronic cancer, we’ll have that data read out probably sometime in mid-2026 (second half) although I’ll be providing enrollment updates in 2025 as we go. So quite a bit of clinical activity. On the public health side, a lot going on there with regard to some of the various things we’re doing; publications in different journals. And so a lot of news flow over the next coming months across both business segments.
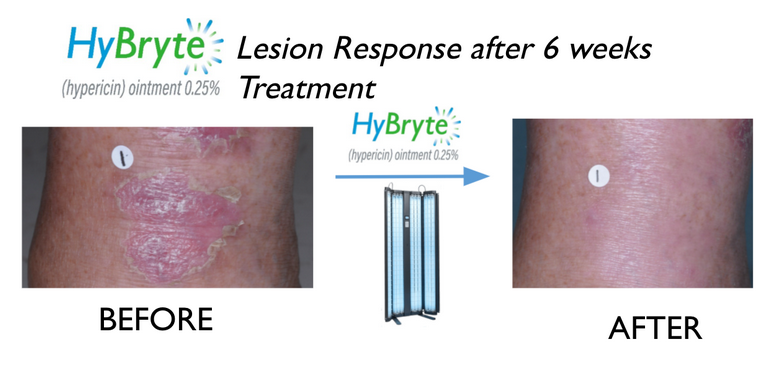
WSA: Yeah. That’s great. And what have been some of the other major developments for HyBryte since we last spoke?
Chris Schaber: So, as I noted, we’ve completed a Phase 3 study , and published it, and we’ll e now be doing a confirmatory Phase 3 study. It’s a study in 80 patients. It’s a double-blind placebo-controlled study that will have sites in the US and Europe where we will be looking to enroll and replicate in many ways what we did in the first Phase 3 study, looking at the same patient population, same primary endpoint and really expanding that overall body of knowledge that we could then turn and submit to the health authorities worldwide.
WSA: Definitely look forward to seeing that coming out here as you mentioned. And so how does your solution and your treatment compare to other treatments and options and studies that are out there for CTCL?
Chris Schaber: I think what makes ours unique is that it has the potential to be the first frontline treatment in this rare disease. Many of the therapies that are out there, are either in development for later stage, more severe disease or commercialized for later stage disease. And not only that, but even those that are used in earlier stage disease are more of a second line therapy after certain therapies have failed. So it could be chemotherapeutic agents that may be approved here, like Mechlorethamine or Valchlor®, or bexarotene or Targretin® is another product. But those are both second line treatments after skin directed therapies have failed; and/or you have off-label use of unapproved therapies, such as photo therapies that use ultraviolet B, ultraviolet A.) So, for early stage cutaneous T-cell lymphoma, it’s a lot of off-label use or use of therapies that quite honestly are more like chemotherapeutics, DNA damaging is their mechanisms.
So, as you would imagine in a disease like Cutaneous T cell lymphoma, which is a chronic cancer — clinicians must manage patients who have lived with it for a long time. The goal in this chronic and difficult to treat disease, is to keep it in its early stages, with the safety of the therapy being just as important as the efficacy.This is where I think HyBryte has the advantage. We’re a topical agent, very safe. We apply it to the lesions on the skin, it follows a different mechanism of action, a mitochondrial pathway versus DNA damaging. We then activate our drug on the skin with safe, visible fluorescent or LED light to shrink the lesions. There’s no cure with this disease, as I noted out earlier, it’s a chronic disease and you need to manage it a lot like you would psoriasis. So with this in mind, the safety–the drug is very benign, the light is very benign, and it’s been well received to date and we now will be doing that confirmatory Phase 3 study to get a little bit more data out there to satisfy some of the health authorities. And that’s what our focus is.
WSA: For sure. And so you had touched on this when you’re speaking about the most recent activity there in news. So can you talk a little bit further about the start of the SGX945 study that’s for the patients with inflamed blood vessels?
Chris Schaber: Yes. SGX945 is a separate molecule from HyBryte and the molecule that we’re looking in psoriasis. SGX945 is an injectable agent called dusquetide. It’s currently administered as a short four minute IV infusion that’s given periodically to patients. You mentioned inflamed blood vessels, but that’s only one aspect of this disease. The disease is actually aphthous ulcers in Behçet’s disease. A rare disease, another chronic disease where there is no cure. An autoimmune disease. And with Behçet’s, what this autoimmune disorder causes is inflammation in multiple parts of the body that is characterized by recurrent oral and genital ulcers, skin lesions and inflammation of the eyes, joints, and blood vessels, as you noted.
This disease usually affects people in their 20s to 30s, and it is more prevalent in the Middle East, Asia and Japan but is a small orphan disease in the United States. So we believe that our SGX945, which is an innate defense regulator, modulates the body’s own innate immune system, and is nicely aligned in treating this disease and the inflammation associated with it. And this is a Phase 2 study that we just initiated earlier this month where we expect top line results in the first half of next year in this small study.
WSA: Great, so what other goals and milestones that perhaps we didn’t cover you’re hoping to accomplish here over the course of the next six to 12 months?
Chris Schaber: Well, we have quite a bit on the clinical data front as I outlined. I think that’s probably the most important, in addition to the initiation of the Phase 3 HyBryte study in cutaneous T-cell lymphoma, with updates along the way as we enroll there. I’ll have an enrollment update in the second half of 2025, and I’ll be able to give further guidance on when that study will complete. We have the psoriasis study. The Phase 2a study in psoriasis that’s ongoing using the same active ingredient as in HyBryte, where we’ll have top line results here in the first half of next year. And then with the Behçet’s study we just spoke of with SGX945, top line results are expected there in the first half of 2025 as well. So those are the things I can talk about. Obviously, we remain active on the business development front. There’s no promises I make there, but we are looking at everything from partnership to M&A; things that could make sense for our shareholders and the company.
We would not announce anything until there was a deal to be had there. And we also continue to pursue government grants and contracts. And when we get those awards, we announce those. There are other things going on in the background as it relates to the other programs, especially in the public health solutions business segment with that funding. And we do have some supportive studies going on in the background with HyBryte as well, where we’ll be periodically announcing data. One in particular is an investigator-initiated study looking at longer durations of treatment with HyBryte — up to one year. This is an investigator-initiated study at the University of Pennsylvania, and we’ve already announced some data, which was well received by the market and by clinicians. And we plan to give updates on that study as it goes.
But, thus far, it really shows the profound effects of HyBryte, with longer durations of treatment. And luckily, thus far, every study we’ve done with HyBryte has been successful, has worked and we’re hoping that that continues. And the interesting thing with HyBryte is because it’s a topical agent where you apply light to activate it, it’s such a direct therapy that you can see the response. And that’s what I think makes this quite a unique molecule and where we sit today in the development path, it’s quite de-risked. Not without risk — all development has risk, obviously, but we’ve de-risked this substantially now in Phase 3 clinical studies.
WSA: Great, so what other goals and milestones that perhaps we didn’t cover you’re hoping to accomplish here over the course of the next six to 12 months?
Chris Schaber: Well, we have quite a bit on the clinical data front as I outlined. I think that’s probably the most important, in addition to the initiation of the Phase 3 HyBryte study in cutaneous T-cell lymphoma, with updates along the way as we enroll there. I’ll have an enrollment update in the second half of 2025, and I’ll be able to give further guidance on when that study will complete. We have the psoriasis study. The Phase 2a study in psoriasis that’s ongoing using the same active ingredient as in HyBryte, where we’ll have top line results here in the first half of next year. And then with the Behçet’s study we just spoke of with SGX945, top line results are expected there in the first half of 2025 as well. So those are the things I can talk about. Obviously, we remain active on the business development front. There’s no promises I make there, but we are looking at everything from partnership to M&A; things that could make sense for our shareholders and the company.
We would not announce anything until there was a deal to be had there. And we also continue to pursue government grants and contracts. And when we get those awards, we announce those. There are other things going on in the background as it relates to the other programs, especially in the public health solutions business segment with that funding. And we do have some supportive studies going on in the background with HyBryte as well, where we’ll be periodically announcing data. One in particular is an investigator-initiated study looking at longer durations of treatment with HyBryte — up to one year. This is an investigator-initiated study at the University of Pennsylvania, and we’ve already announced some data, which was well received by the market and by clinicians. And we plan to give updates on that study as it goes.
But, thus far, it really shows the profound effects of HyBryte, with longer durations of treatment. And luckily, thus far, every study we’ve done with HyBryte has been successful, has worked and we’re hoping that that continues. And the interesting thing with HyBryte is because it’s a topical agent where you apply light to activate it, it’s such a direct therapy that you can see the response. And that’s what I think makes this quite a unique molecule and where we sit today in the development path, it’s quite de-risked. Not without risk — all development has risk, obviously, but we’ve de-risked this substantially now in Phase 3 clinical studies.
WSA: Certainly. And perhaps you can walk us through your background experience, Chris, and that of your key management team. How are your interests aligned with shareholders and potential investors?
Chris Schaber: I’ve been in drug development in the Pharma/biotech industry for close to 35 years now. I started in big pharmaceutical companies and then left to start and build and grow biotech. I had a nice exit from a company that I helped co-found and then came to Soligenix to build out the pipeline that we have today. And as you typically do when you have a long career, the folks I worked with in previous lives, previous companies that we’ve had some success, I’ve taken some along with me and they’re working with me here at Soligenix. Most notably my Chief Medical Officer, Dr. Richard Straube. And we’re looking to do it again, and I think we’re on the right path. We have some work to do, but we’ve really done a good job with the clinical studies, especially with HyBryte in cutaneous T-cell lymphoma, and are looking to get our lead candidate over the finish line.
But again, this is something I’ve been doing for many years. I’ve been passionate about most of my career in the rare disease area and the unmet medical needs setting. What we’re looking to do first and foremost, is help patients. As you know, when you are successful in helping patients along the way, comes the success with the company, with the market, etc. And that’s what we’re looking to do, is continue to build on that foundation to increase the value of the company for shareholders as well as advance therapies that can help a number of people.
WSA: So yeah. Right now current share price $3.41 a share market cap about 9 million US. So before we conclude here, Dr. Schaber, why do you believe investors should consider the company as a good investment opportunity today?
Chris Schaber: I think if you’re looking at the biotech drug development space, you obviously understand it a little bit. I think one thing to point out is our cap structure is relatively clean, which is always a good thing for small startup biotech companies, as well as the status or phase of the pipeline. Given the fact that we have a number of studies that have already demonstrated biologic activity, proof of concept, and ultimately, as I stated with our lead candidate, HyBryte in cutaneous T-cell lymphoma have a second confirmatory phase three study. It’s already shown success, both efficacy and safety in multiple clinical studies prior to this one. We have a confidence level going in that we have the potential to be quite successful here, and that’s where our focus and drive and passion lies. And I think if you’re looking at this type of space, I think Soligenix is one that you should look at.
WSA: Well, we certainly look forward to continuing to track the company’s growth and report on the upcoming progress. And we’d like to thank you for taking the time to join us today there, Chris, and update our investor audience. It was great having you on.
Chris Schaber: My pleasure. Thank you for the time and for allowing me to speak a little bit about Soligenix. I will point out that we have a lot of good information on our website, at www.soligenix.com, where you can find a lot of additional information about the company, the program pipeline, etc. So, I would urge folks if they’re interested, to look there as well.
WSA: Great. Yeah, we’ll make sure we post a link here on our site as well.
Chris Schaber: Thank you.
